Clotrimazole for Vulvovaginal Candidosis: More Than 45 Years of Clinical Experience
Authors: Werner Mendling, Maged Atef El Shazly and Lei Zhang
Abstract
Vulvovaginal candidosis is a common disease, and various treatment strategies have emerged over the last few decades. Clotrimazole belongs to the drugs of choice for the treatment of vulvovaginal candidosis. Although available for almost 50 years, systematic reviews on the usefulness of topical clotrimazole across disease severity and populations affected are scarce. Thus, we conducted a systematic literature search in the PubMed and Embase databases to summarize the effectiveness and safety of topical clotrimazole in the treatment of uncomplicated (acute) and complicated vulvovaginal candidosis. In total, 37 randomized controlled studies in women suffering from vaginal yeast infections qualified for inclusion in our review. In women with uncomplicated vulvovaginal candidosis, single intravaginal doses of clotrimazole 500 mg vaginal tablets provided high cure rates and were as effective as oral azoles. A single dose of clotrimazole 500 mg was equipotent to multiple doses of lower dose strengths. Prolonged treatment regimens proved to be effective in severe and recurrent cases as well as in symptomatic pregnant women. It is therefore expected that in the general population, clotrimazole will continue to be widely used in the field of vaginal health in the upcoming years; more so as clotrimazole resistance in vaginal candidosis is rare.
Keywords: canesten; clotrimazole; vulvovaginal; vaginitis; mycosis; candidosis; yeast infection; candida; candida albicans; vaginal health
1. Introduction
Clotrimazole is an imidazole antimycotic agent that was discovered in the 1960s. It has a specific chemical structure consisting of four aromatic rings, out of which one represents an imidazole ring [1]. Clotrimazole has a broad antimicrobial activity against Candida albicans and other fungal species. Like other azole-type antifungal drugs, the antimycotic properties are mediated by an interaction with ergosterol synthesis (via inhibition of the fungal cytochrome 14α-demethylase enzyme) eventually resulting in increased fungal cell wall leakiness with disruption of the structure and function of the cell wall [2]. Topical clotrimazole is widely used for the treatment of tinea pedis (athlete’s foot), cutaneous mycoses, and oropharyngeal candidosis [1,3]. It also belongs to the drugs of choice for the topical treatment of vulvovaginal candidosis and Candida balanitis [4,5].
Clotrimazole was first registered as Canesten® in Germany more than 45 years ago (in 1973) [6]. The initial formulation for local treatment of vulvovaginal candidosis was the vaginal tablet [7] followed by internal vaginal cream, external cream and soft ovule (soft capsule). Additional formulations are marketed under other trade names. Drug combinations (e.g., clotrimazole plus fluconazole) are also available nowadays. Clotrimazole monopreparations for the management of vulvovaginal candidosis are available over the counter in most countries and cover a dose range from 100 to 500 mg (solid systems). Comparable local clotrimazole exposure can be achieved by administration of semi-solid systems (e.g., creams containing clotrimazole 1%, 2% or 10%) to the vagina and vulva [1]. While many preparations are available as generics, Canesten® is still the market leader in this field [8]. Clotrimazole has a poor oral bioavailability. When administered intravaginally, approximately 3% of the dose is systemically available [9]. The latter explains the favorable systemic tolerability of clotrimazole following vaginal application. Approximately 70–75% of childbearing aged women experience symptomatic vulvovaginal candidosis at least once during their life and 40–50% will suffer from repeated episodes during their lifetime. About 5–8% of adult women may experience recurrent vulvovaginal candidosis (i.e., ≥4 episodes per year) [10,11]. Candida albicans is the most common pathogen, but other non-albicans Candida species may also be causative microorganisms, particularly in association with disease recurrence [8,11]. The treatment of vulvovaginal candidosis depends on whether the infection is uncomplicated or complicated. Complicated cases comprise recurrent, severe and non-albicans vulvovaginal candidosis as well as vulvovaginal yeast infections during pregnancy and in subjects with immunocompromised conditions [4]. According to current treatment guidelines, topical clotrimazole may play a role in the treatment of both uncomplicated and complicated cases [4,12–14]. Despite the fact that in certain patient subpopulations resistance to clotrimazole has been reported [1,15], clotrimazole resistance in vaginal candidosis is rare and susceptibility testing is usually not recommended [12,16]. It is therefore expected that in the general population, clotrimazole will continue to be widely used in the field of female intimate health in the upcoming years.
This article presents the results of a systematic literature search on the effectiveness and safety of topical clotrimazole when used for the treatment of uncomplicated (acute) and complicated vulvovaginal candidosis. In addition, the scientific evidence for its topical use in men with Candida balanitis will also be explored.

3.2. Clotrimazole in Complicated Vulvovaginal Candidosis
3.2.1. Vaginal Yeast Infection during Pregnancy
During pregnancy, up to 50% of women experience vulvovaginal candidosis [51]. In addition, in pregnant women, recurrent vaginal yeast infections and insufficient therapeutic responses are more frequently recorded than in non-pregnant women [10,52]. An increased content of glycogen and lower pH value in the vagina as well as an intensified binding of yeast cells to the vaginal mucosa have been made accountable for this observation [10,51]. During pregnancy, the therapy of symptomatic vaginal yeast infections should be intense, but restricted to topical preparations; oral antifungals should be avoided [10,14]. Specifically, topical azoles can be used at all stages of pregnancy because there is no or only minimal systemic exposure following intravaginal administration [53]. The FDA assigned topical clotrimazole to pregnancy category B. The other topical imidazoles and triazoles have been assigned to category C. In fact, several clinical trials confirmed the safety of clotrimazole in pregnancy; no association was observed between vaginal application of clotrimazole and congenital abnormalities [54].
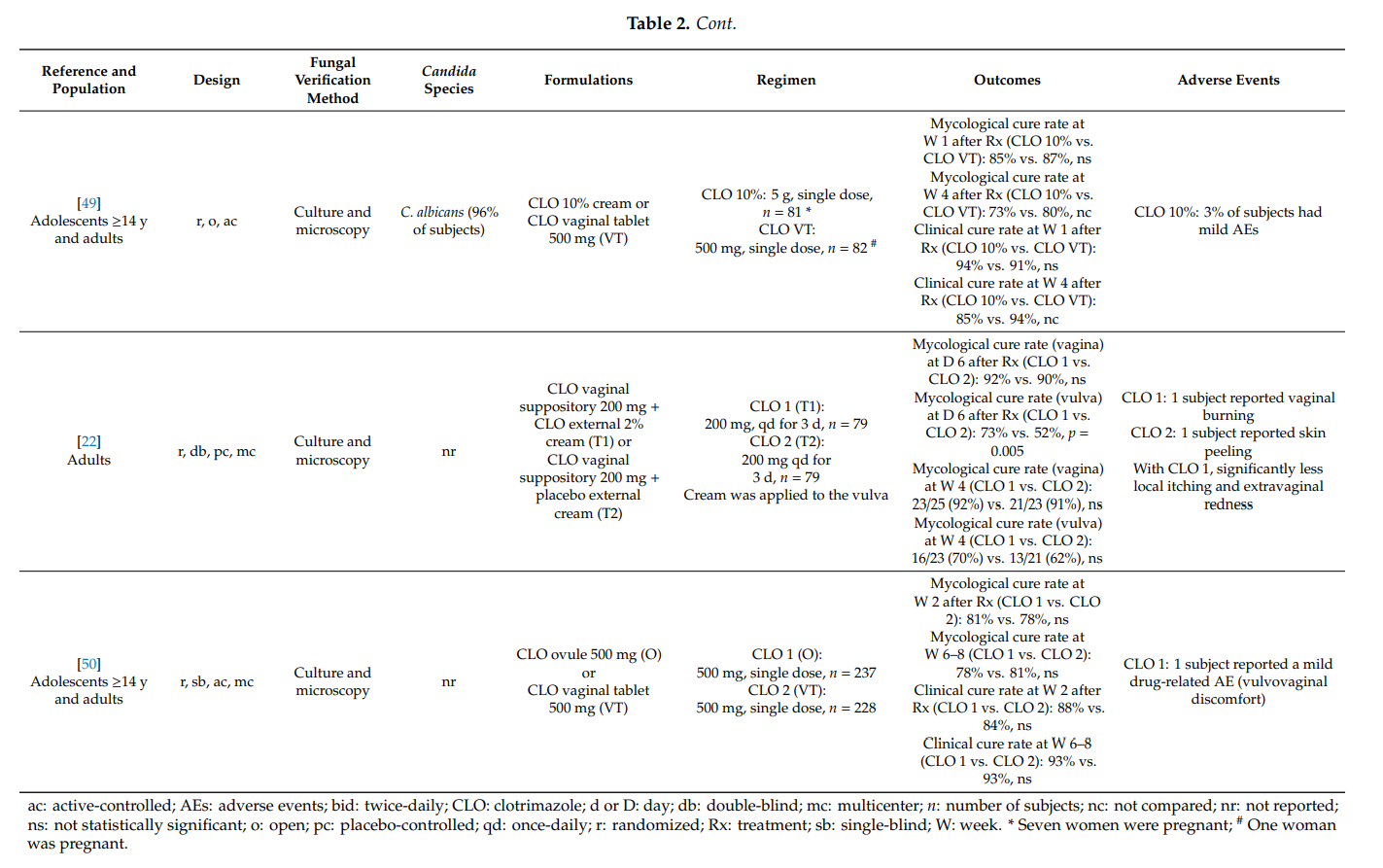
Our systematic literature search identified five articles which qualified for inclusion (Table 3). In pregnant women with symptomatic vulvovaginal candidosis, clotrimazole 100 mg (vaginal tablet), administered for approximately 1 week, provided high mycological cure rates (78–88% at 1 or 2–4 weeks after therapy). Similar results were observed following 1-week application of clotrimazole 1% internal cream. Intravaginal clotrimazole was significantly more effective than placebo treatment [55]. In the same and other studies, the prophylactic use of clotrimazole during pregnancy significantly lowered the frequency of Candida presence on the neonatal skin [55–59]. Moreover, mycological cure rates were markedly higher with multiple-dose clotrimazole compared with nystatin, while there was no difference to terconazole. There was a trend towards reduced effectiveness of clotrimazole when taken as a single intravaginal 500 mg dose. In all five studies, topical clotrimazole was well tolerated.
Two additional randomized controlled trials studied the potential of clotrimazole in the prevention of preterm birth when administered to pregnant women with asymptomatic vaginal candidosis. Kiss et al. [60] evaluated whether a screening strategy in pregnant women presenting for their prenatal visits early in the second trimester lowers the rate of preterm delivery. Subjects with pathological vaginal flora were randomized to an intervention group or control group (no treatment). Women who received intravaginal clotrimazole 100 mg once-daily for 6 days due to asymptomatic vulvovaginal candidosis showed a significantly lower spontaneous preterm birth rate than untreated women (8/294 (2.7%) vs. 22/292 (7.5%); risk ratio (RR): 0.36; 95% confidence interval (CI): 0.16, 0.80) [61]. In a smaller study conducted by Roberts and colleagues [62], 98 pregnant women at <20 weeks of gestation were randomized to intravaginal clotrimazole 100 mg once-daily for 6 days or no treatment. There was a reduction in preterm birth rate in women treated with clotrimazole (4.0% vs. 6.3%), but the difference did not reach statistical significance. A meta-analysis of both trials concluded that
treatment of asymptomatic candidosis may reduce the risk of preterm birth, but further prospective, sufficiently powered studies are required [61].
Our findings are in agreement with a previous systematic review on the topical treatment of symptomatic vulvovaginal candidosis in pregnancy. A Cochrane review from 2001 [63] concluded that topical imidazoles are more effective than nystatin and should be used during pregnancy. In addition, short-term treatments with topical imidazole drugs were found to be less effective than 7-day administrations, and topical treatments beyond 7 days provided no additional benefit.
3.2.2. Recurrent Vaginal Yeast Infection
Recurrent symptomatic vaginal yeast infections may have a severe adverse impact on the quality of life. Defined as ≥4 culture-proven episodes per year, it is a long-term condition causing significant morbidity in women [68]. The highest prevalence (9%) has been observed in young women aged 25–34 years [8]. The main fungal species causing recurrent vulvovaginal candidosis is still Candida albicans. In fact, in about 85–95% of cases, azole-sensitive Candida albicans can be identified as the responsible pathogen. The latter implies that peculiarities of the host (e.g., genetic factors that facilitate vaginal colonization and persistence) must contribute to the development of disease recurrence [68]. Optimized treatment regimens are required to combat this debilitating and complex disease. Usually, an induction course followed by maintenance or intermittent treatment for at least 6 months is pursued [14].
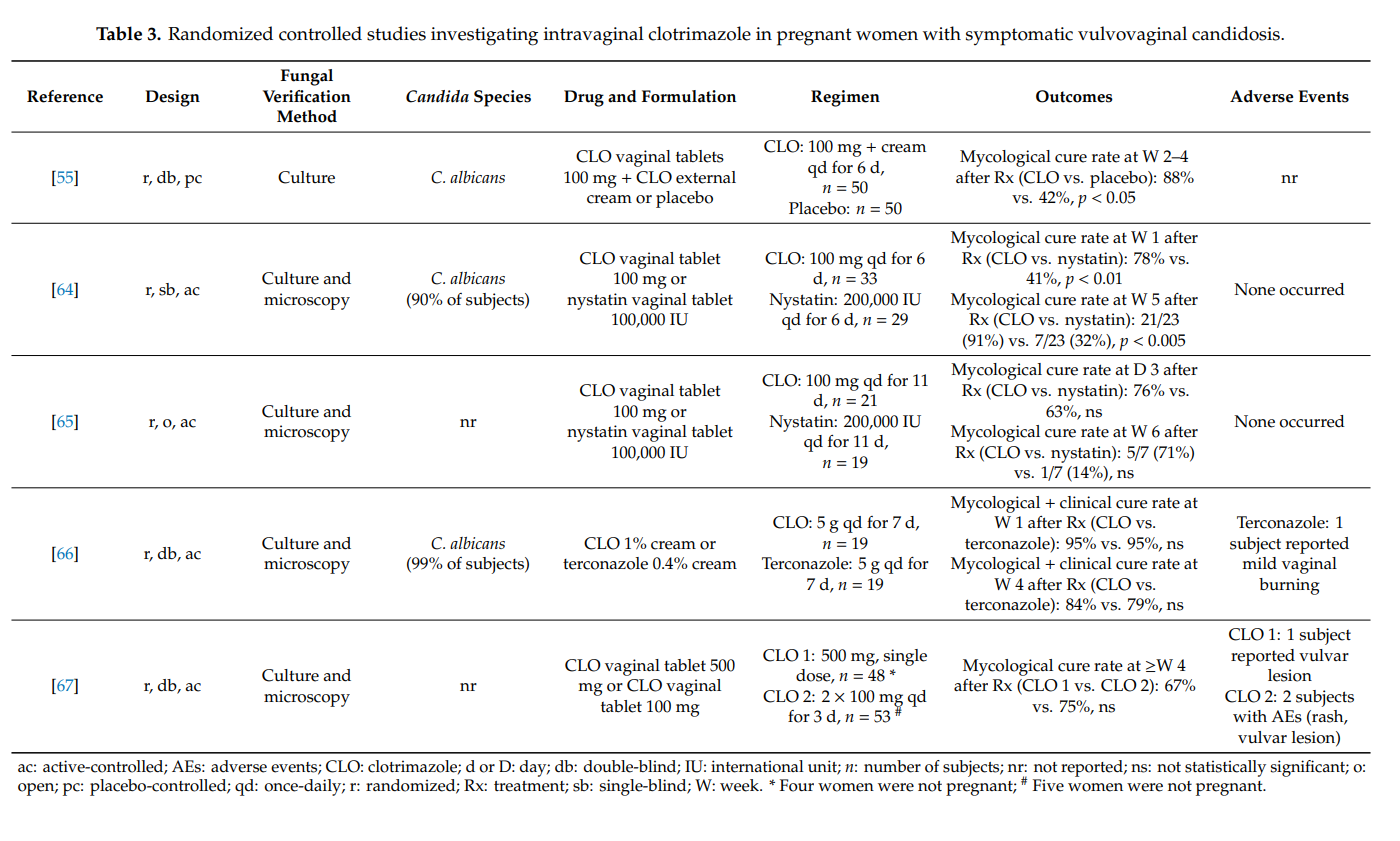
We found four randomized controlled studies investigating intravaginal clotrimazole in non-pregnant women with recurrent symptomatic vulvovaginal candidosis (Table 4). Clotrimazole induction treatment for 1–2 weeks (e.g., clotrimazole 100 mg once-daily) resulted in short-term mycological and clinical cure rates of >80%. Intermittent once-monthly prophylactic administration of clotrimazole 500 mg (solid system) for 6 months prevented clinical disease recurrence in up to 70% of women, but did not prevent vaginal recolonization with Candida albicans in the majority of cases. More subjects on intermittent intravaginal clotrimazole remained asymptomatic than subjects receiving intermittent oral itraconazole or placebo. In one head-to-head study, clotrimazole and oral ketoconazole induction treatments were equally effective at achieving high short-term cure rates, but without immediate initiation of maintenance/intermittent therapy, longer-term recurrence rates were high in both groups [69]. Except for occasional vulvovaginal burning, topical clotrimazole was well tolerated in all four studies. Adverse events were significantly more common with oral itraconazole and oral ketoconazole than with intravaginal clotrimazole.
Our findings summarized in Table 4 must be put into perspective with the fact that higher cure rates have been reported with oral fluconazole. In a randomized controlled study in 387 women with recurrent symptomatic vulvovaginal candidosis, oral fluconazole 150 mg was administered on days 0, 3, 6, followed by once-weekly intakes for 6 months. The proportion of women who remained clinically cured until 6 months was 91% in the fluconazole group and 36% in the placebo group (p < 0.001). During the maintenance phase, 2.9% of patients in the fluconazole group and 1.2% in the placebo group reported at least one adverse event that led to discontinuation of the study medication [70].
3.2.3. Severe Vaginal Yeast Infection
Severe vulvovaginal candidosis is characterized by extensive vulvar erythema, swelling, excoriation, itching and fissure formation. Standard treatments as used in uncomplicated vulvovaginal candidosis are insufficient in women with severe disease; intensified treatment regimens comprising repeated doses are required [4,10,14].
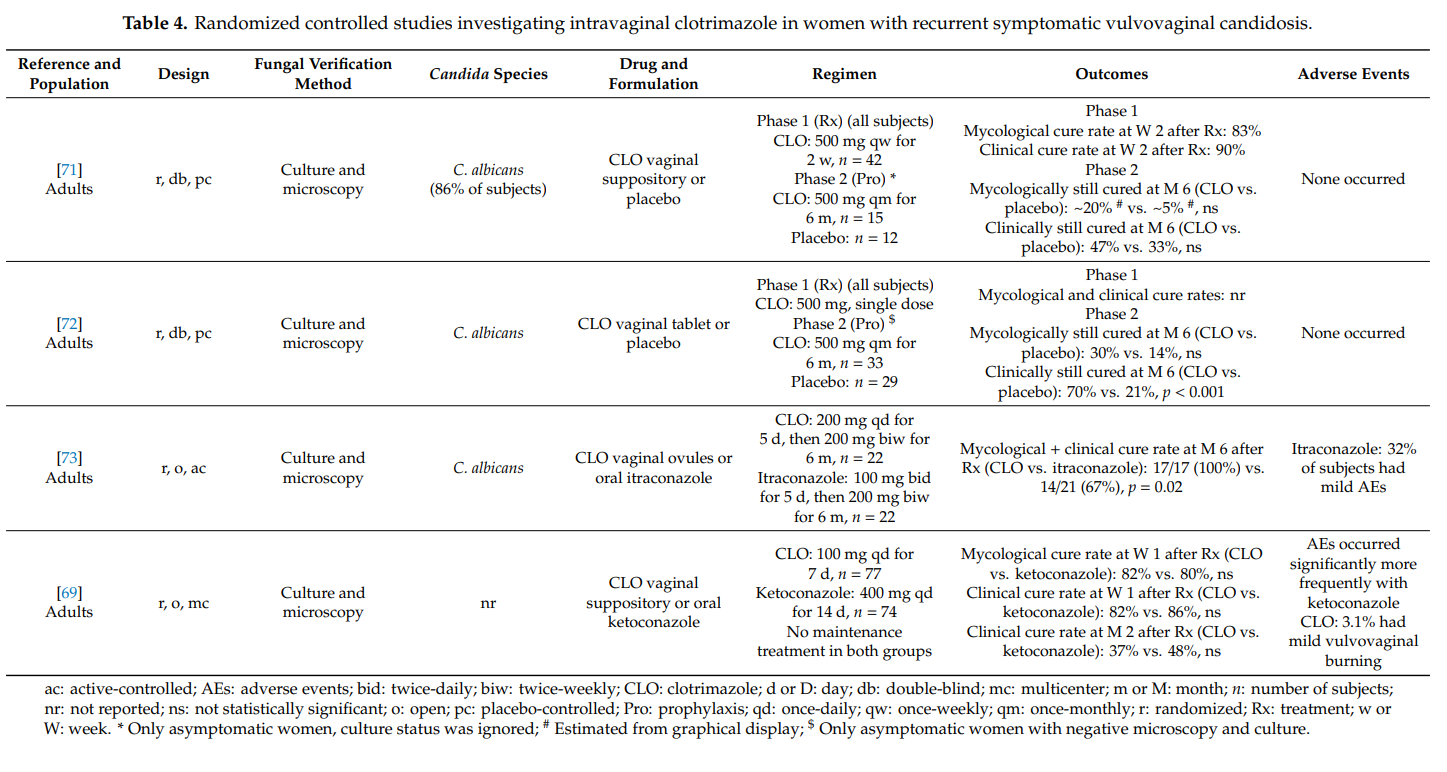
We identified one article qualifying for inclusion in our review. Zhou et al. [74] conducted a prospective, open, randomized (1:1) study in 240 women with severe vulvovaginal candidosis to assess whether two 500 mg doses of clotrimazole vaginal tablet are as effective as two 150 mg doses of oral fluconazole. In each group, the two doses were administered 3 days apart. Study participants were to be not pregnant and at least 18 years old. In 90% of cases, Candida albicans was the causative pathogen. The mycological cure rates at days 7–14 after therapy were 78% and 74% (p = 0.147) in the
clotrimazole group and the fluconazole group, respectively. The corresponding clinical cure rate was 89% in both groups. At days 30–35, the mycological cure rates amounted to 54% and 56% (p = 0.813) in the clotrimazole group and the fluconazole group, respectively; the clinical cure rates at that time point were 72% and 78% (p = 0.298), respectively. Systemic adverse events were more common with oral fluconazole (e.g., headache) than with intravaginal clotrimazole, while local adverse events (e.g., mild burning) occurred more frequently in the clotrimazole group. It was concluded that the two tested treatment regimens are equally effective, with a safety benefit for clotrimazole.
3.2.4. Vaginal Yeast Infection in Immunocompromised Host
Vulvovaginal candidosis is of particular concern in immunocompromised subjects such as women with poorly controlled diabetes mellitus, HIV infection, or on immunosuppressive drugs. Usually, these populations require prolonged conventional antifungal therapy for 7–14 days, including intravaginal azole therapy [4,11,14].
We found no qualifying study that investigated the therapeutic effectiveness of intravaginal clotrimazole in this setting. However, Williams and colleagues performed a randomized, double-blind,
placebo-controlled study on the potential of intravaginal clotrimazole to prevent vaginal candidosis in adult women with HIV [75]. In the clotrimazole arm, study participants applied the drug (capsules containing clotrimazole powder 100 mg) once a week. At 6-month intervals, vaginal samples were collected and examined. The risk of experiencing an episode of vulvovaginal candidosis during clotrimazole use was significantly reduced compared to placebo (risk ratio: 0.4; 95% CI: 0.2, 0.9].
3.2.5. Non-Albicans Vaginal Yeast Infection
In about 10% of cases, non-albicans Candida species are responsible for acute symptomatic vulvovaginal candidosis [14]. Among them, Candida glabrata is the most frequently identified strain [68]. Non-albicans Candida species as causative pathogens of vulvovaginal candidosis are an emerging threat. Risk factors include uncontrolled type 2 diabetes and advanced age as well as intake of glycosuria-inducing agents to manage type 2 diabetic patients [68]. The optimal treatment of non-albicans vaginal yeast infections has not been established [4]. Non-albicans Candida infections are unlikely to respond to standard treatments. In fact, azoles are poorly effective in the treatment of Candida glabrata-caused vulvovaginal candidosis [10]. Boag and co-workers [76] investigated the effect of oral fluconazole and intravaginal clotrimazole on the vaginal microbial flora. The vaginal flora was unaltered after both therapies. In women suffering from vaginitis due to Candida glabrata or Candida krusei, the yeasts persisted longer and revealed a poorer treatment response to either treatment. Our literature search did not identify qualifying clotrimazole-studies in this setting.
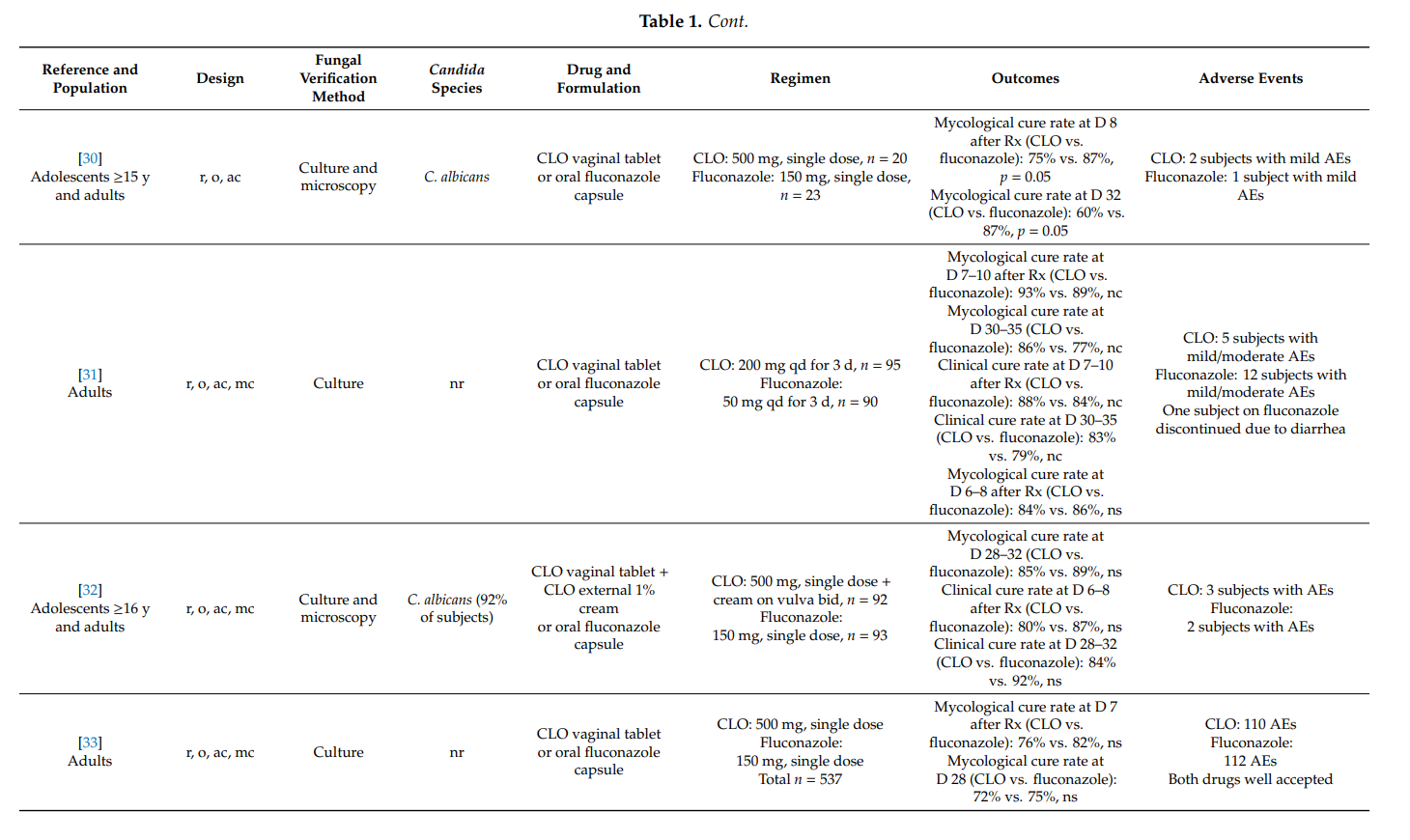
3.3. Clotrimazole in Men with Candida Balanitis
Balanitis affects approximately 3–11% of men at least once during lifetime.
Fungal microorganisms—especially Candida albicans—are most frequently responsible for this itching inflammation of the glans penis [5]. Candida balanitis is not considered a sexually transmitted disease, but in approximately 20% of male partners of women with recurrent vulvovaginal candidosis, Candida species can be isolated on their penises [10]. In one study, 43% of men with Candida balanitis had partners with Candida vaginitis [77]. In another study, 107 male partners of women with acute vaginal candidosis were examined, and 45% exhibited symptoms of balanitis [48]. Those men who develop Candida balanitis benefit from topical antifungal treatment [4].
In a randomized (1:1), open-label study, Stary et al. [77] compared the effectiveness and safety of a single oral dose of fluconazole 150 mg with clotrimazole 1% external cream applied topically twice-daily for 7 days in 157 adult men with balanitis. In those study participants with a positive baseline culture for Candida albicans, 49/63 (78%) subjects in the fluconazole group and 53/64 (83%) subjects in the clotrimazole group were mycologically cured at days 8–11 after initiation of therapy (p > 0.05). At the same time-point, 92% and 91% (p > 0.05), respectively, were clinically cured or had improved. At the one-month follow-up visit, 26/36 (72%) subjects in the fluconazole group and 25/33 (76%) subjects in the clotrimazole group were mycologically cured. The median time to relief of symptoms (erythema) was 6 days during fluconazole treatment and 7 days with clotrimazole. Both treatments were well tolerated. It was concluded that both treatments are equipotent in the treatment of Candida balanitis.
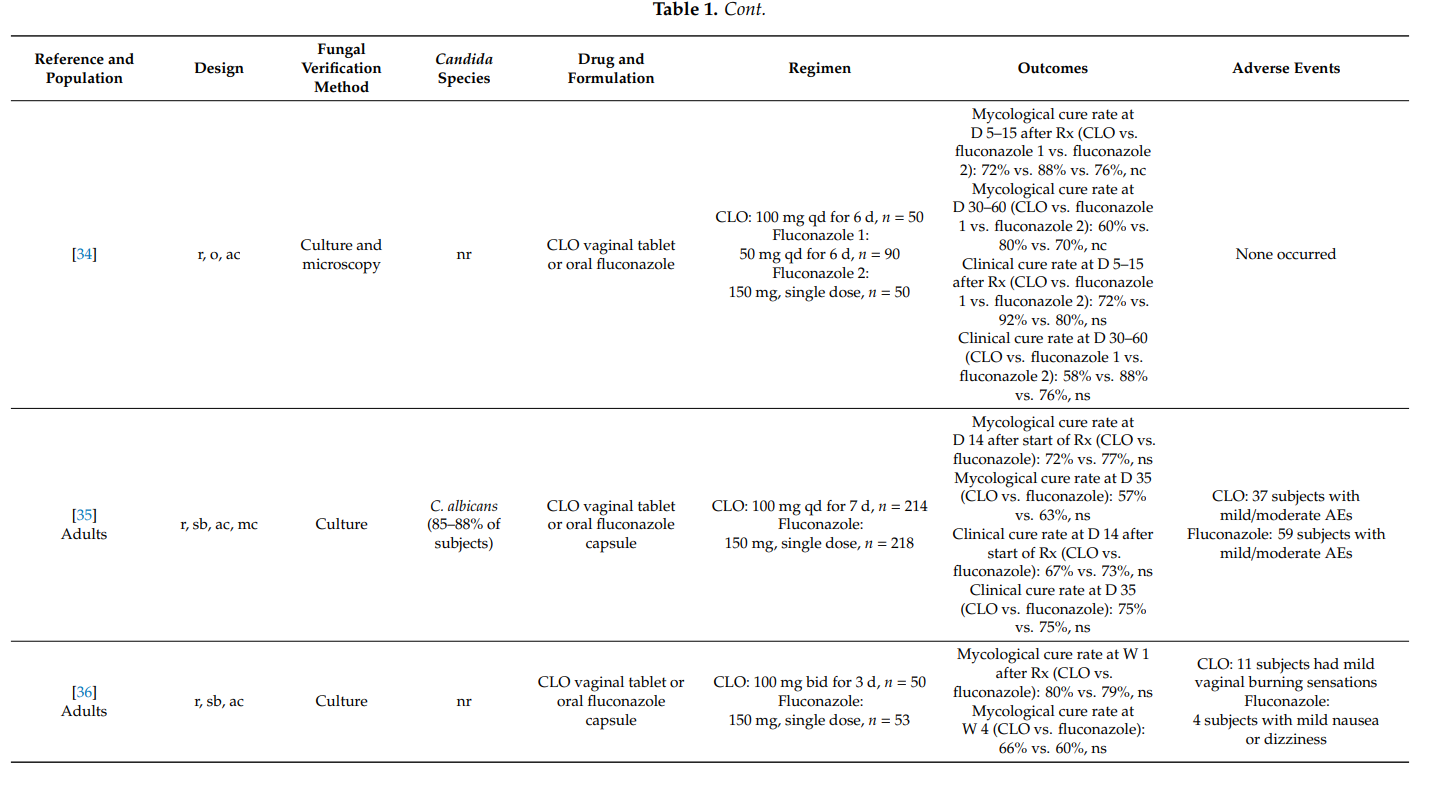
Maw et al. [78] conducted an open, comparative study of bifonazole and clotrimazole in adults with candidal balanoposthitis (i.e., both the glans and the foreskin were affected). Study participants were randomized to receive either bifonazole 1% cream once-daily for 6 days or clotrimazole 1% cream twice-daily for 6 days. On day 7 after treatment initiation, 21/27 (78%) subjects in the bifonazole group and 20/26 (77%) subjects in the clotrimazole group were mycologically cured. The difference was not statistically significant. No adverse events were reported in the clotrimazole group while one subject in the bifonazole group reported increased erythema following initial application.
In an uncontrolled study in 138 men with Candida balanitis, clotrimazole 1% external cream applied topically twice-daily for 7 days yielded mycological cure rates of 90% at day 7 after the start of therapy. Treatment was acceptable even to subjects who continued to engage in sexual activity during treatment [79].
4. Summary
More than 45 years ago, the first topical clotrimazole formulation (Canesten® vaginal tablet) was registered. Today, it is available in different dosages and formats allowing treatments according to individual needs and preferences. Various studies and its therapeutic use over decades confirmed the antimycotic activity of clotrimazole in the treatment of vulvovaginal candidosis. Despite its use over many years, clotrimazole resistance in vaginal candidosis is rare, with the caveat that drug resistance has emerged particularly in immunocompromised patients [1,12]. Resistance has been associated with the overexpression of efflux pump genes. Changes in the clotrimazole target, the fungal cytochrome 14α-demethylase enzyme, may also play a role in some cases [1]. We conducted a systematic literature search on the effectiveness and local tolerability of topical clotrimazole when used for the treatment of uncomplicated and complicated vulvovaginal candidosis, and when used topically by men with Candida balanitis. In total, 39 randomized controlled studies qualified for inclusion in our review.
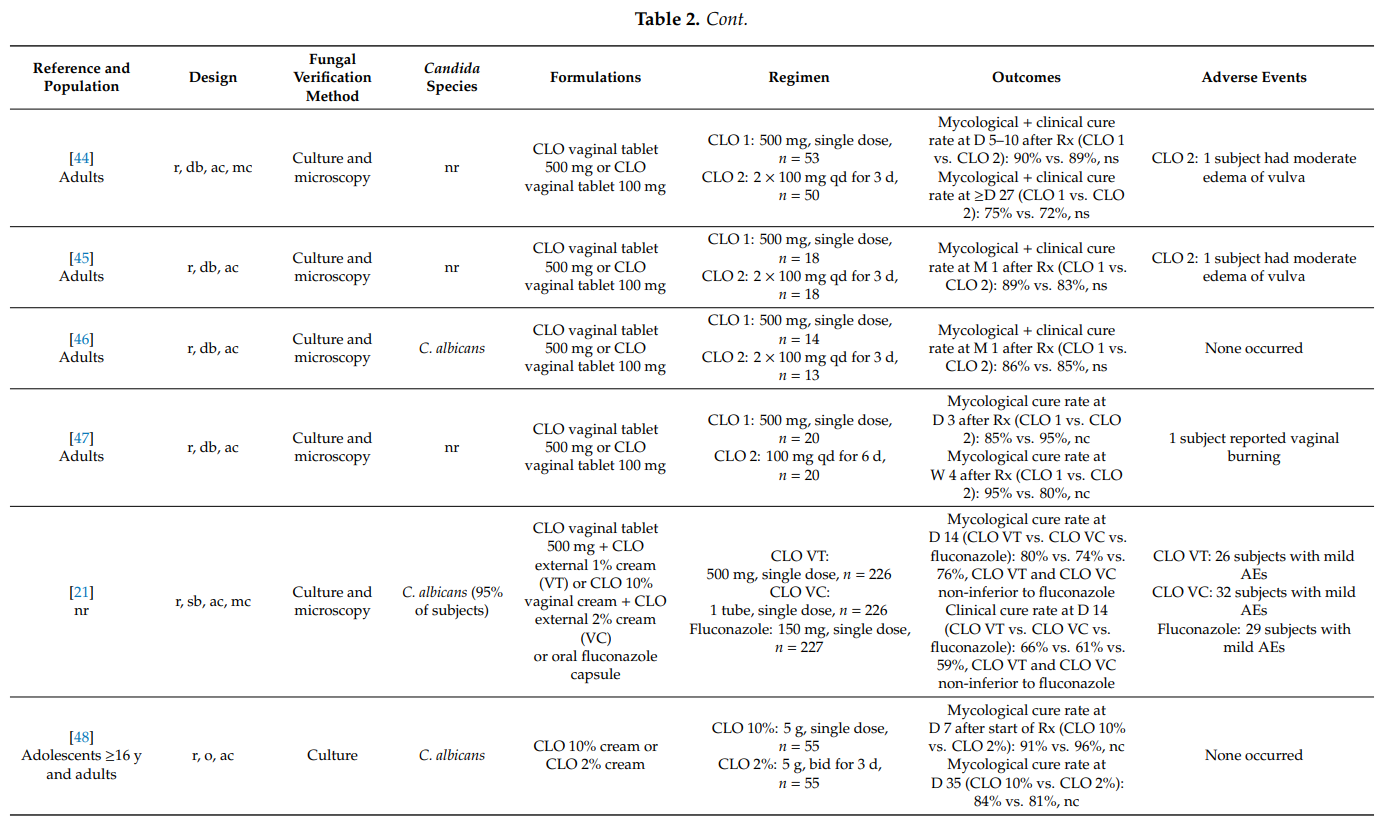
In women with uncomplicated (acute) vulvovaginal candidosis, single topical doses of a clotrimazole 500 mg vaginal tablet provided mycological cure rates of up to 95%. The single use of clotrimazole 10% internal cream or clotrimazole 500 mg ovule resulted in similar results. This is in accordance with the observation that fungicidal concentrations of clotrimazole could be determined in vaginal secretions up to three days after insertion of one vaginal 500 mg tablet [80]. In head-to-head comparative studies, single-dose regimens of intravaginal clotrimazole and oral antifungals were equally effective. In cases when the candidosis extended to the vulvar region, the combined use of intravaginal clotrimazole and external clotrimazole cream provided added benefit (e.g., less local itching). In fact, two observational studies including over 5800 women with vulvovaginal mycosis showed that more than 75% of physicians prefer to treat their patients with a combination of clotrimazole to be applied intravaginally (vaginal tablet or cream) and clotrimazole cream to be applied externally to the vulva and surrounding areas [19,81].
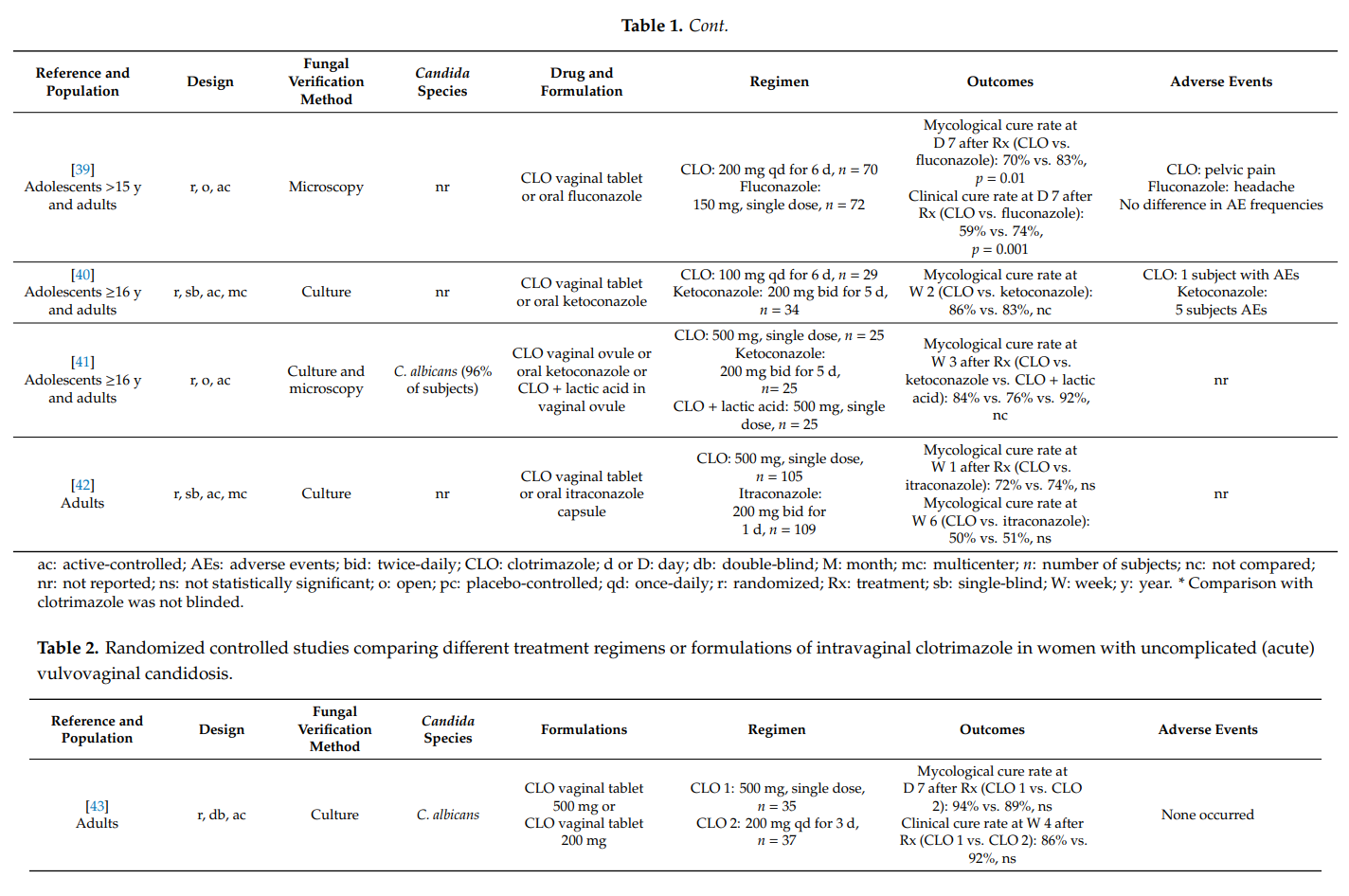
Topical clotrimazole has also proved its effectiveness in the therapy of complicated vulvovaginal candidosis. In pregnant women with symptomatic vaginal yeast infection, one-week treatments with clotrimazole 100 mg or 1% internal cream were associated with high mycological cure rates. Furthermore, the prophylactic use of clotrimazole during pregnancy significantly lowered the risk for Candida infections of newborns and preterm birth.
In recurrent symptomatic vulvovaginal candidosis, clotrimazole induction treatment for 1–2 weeks resulted in short-term mycological cure rates of >80%, and topical treatments applied intermittently (monthly) prevented disease recurrence and breakthrough vaginitis in up to 70%. However, higher clinical cure rates were observed with once-weekly oral fluconazole. In one head-to-head comparative study in women with severe vulvovaginal candidosis, two 500 mg doses of clotrimazole vaginal tablet, administered 3 days apart, were as effective as two 150 mg doses of oral fluconazole, whereas intravaginal clotrimazole was better tolerated. No high-quality studies were found on the usefulness of topical clotrimazole in the therapy of non-albicans vaginal yeast infections and in the treatment of vulvovaginal candidosis in immunocompromised hosts.
Results from two studies provided convincing scientific evidence that clotrimazole 1% external cream, applied topically twice-daily for one week, is associated with high mycological cure rates in men with Candida balanitis. In fact, the latter regimen was as effective as a single oral dose of fluconazole 150 mg.
In all studies, topical clotrimazole was well tolerated; mild local adverse events (e.g., burning) were reported occasionally.
Based on our findings, principal clotrimazole treatment regimens can be inferred. Those are summarized in Table 5.
The results of our literature search are also mirrored in several treatment guidelines. In the guideline issued by the U.S. Centers for Disease Control and Prevention (CDC), it reads: “Short-course topical formulations (i.e., single dose and regimens of 1–3 days) effectively treat uncomplicated vulvovaginal candidosis. The topically applied azole drugs are more effective than nystatin.” [4]. In various other treatment guidelines, short course intravaginal clotrimazole (i.e., vaginal tablet 500 mg as a single dose or 200 mg once-daily for three days) belongs to the recommended regimens for treating uncomplicated vulvovaginal candidosis [12,14,74]. In the World Health Organization (WHO) guideline on the management of vaginal discharge, it reads: “The Guidelines Group recommends that the current best treatment for candida in pregnant women are topical azole preparations. Topical azoles can be used at any stage of pregnancy for treatment of symptomatic candidosis” [14]. This is in agreement with other guidelines [4,12]. In terms of recurrent vulvovaginal candidosis, the most frequently recommended regimen consists of an induction course and maintenance therapy with fluconazole (weekly for six months) [13]. However, in cases when this regimen is not feasible, intermittent topical treatments are also suitable options [4,14]. The American College of Obstetricians and Gynecologists suggests a maintenance therapy with topical clotrimazole 500 mg once-weekly if affected women are “unable or unwilling” to take oral fluconazole [13]. Specific induction therapies can be found in the Canadian Guidelines. Among the recommended regimens, there is oral fluconazole 150 mg, once every 72 h for three doses, and a topical azole for 10–14 days. Maintenance therapy may consist of oral fluconazole 150 mg once-weekly or clotrimazole 500 mg intravaginally once-monthly [82]. According to the CDC, severe vulvovaginal candidosis should be treated with a topical azole for 7–14 days or with two oral doses of fluconazole 150 mg (administered three days apart) [4]. Of note, two doses of clotrimazole vaginal tablet 500 mg (administered three days apart) were shown to be as effective as two doses of oral fluconazole 150 mg, suggesting that both regimens represent suitable treatment options in subjects with severe vulvovaginal candidosis [74].
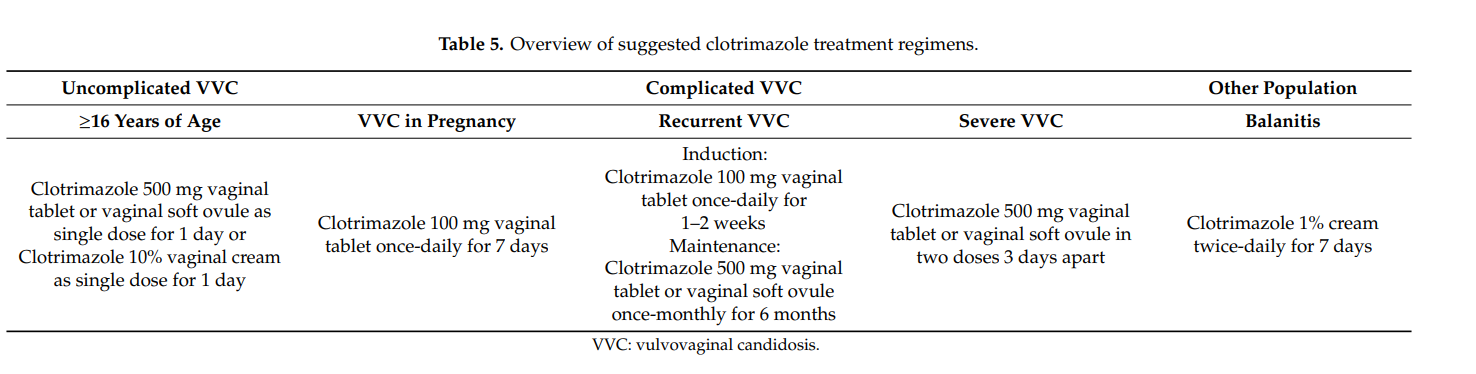
For the treatment of balanitis/balanoposthitis, the WHO recommended topical application of clotrimazole twice-daily for 7 days or nystatin [83]. In fact, imidazoles such as topical clotrimazole 1% twice-daily belong to the drugs of choice in the treatment of Candida balanitis [5].
5. Conclusions
Almost 50 years ago, the first topical clotrimazole formulation was registered for the local treatment of vulvovaginal candidosis. Since then, different formulations have been developed and tested in a variety of studies. Cumulative evidence from numerous randomized controlled trials provided convincing scientific evidence that topical clotrimazole is effective and safe in the therapy of uncomplicated (acute) and certain types of complicated vaginal yeast infections, as well as Candida balanitis. The drug still belongs to the first-line treatments in these settings as specified in several treatment guidelines. It is therefore expected that clotrimazole will continue to be widely used in the field of vaginal health in future, more so as clotrimazole resistance in vaginal candidosis occurs rarely, despite its global use over many years.
Author Contributions: Writing, review and editing: W.M., M.A.E.S. and L.Z.; W.M. provided substantial contribution to the conception of this work and the interpretation of study data. All authors have read and agreed to the published version of the manuscript.
Funding: Writing assistance was provided by Edgar A. Mueller, 3P Consulting and has been funded by Bayer Consumer Care AG, Basel, Switzerland.
Conflicts of Interest: W.M. has received honoraria for oral presentations at scientific congresses or meetings of experts. M.A.E.S. and L.Z. are employees of Bayer Consumer Care AG, Basel, Switzerland.